MONDAY, NOVEMBER 4, 2013 —
Children across Zimbabwe’s Matabeleland region are not being treated for common infections under a recently launched government health programme because the program was not properly publicized. That charge from parents, who say they have reservations about drugs the ministry wants children to take as part of treatments for bilharzia and intestinal worms.
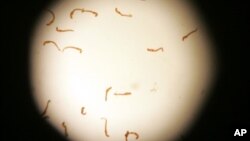
The Ministry of Health and Child Care last week embarked on a mass drug administration exercise, hoping to distribute about 11.5 million donated praziquantel tablets to treat 4.3 million children for bilharzia and intestinal worms. The ministry hoped to be able to reach all children aged 1 to 15 in all of Zimbabwe’s 63 districts.
But some parents declined to let their children take the drugs, saying they needed more information about the drug and any possible side effects. They charge that they had received no information about the treatment program, learning about it only when ministry officials began showing up at area schools.
One Bulawayo parent, Emily Ndlovu, says she did not allow her son to have the drug administered to him at his school. She said she was not comfortable with her son taking praziquantel, mistakenly believing that the drug is banned in the UK. While the drug is not banned, it is true that it is available in the UK and the US only when prescribed by a doctor. It is available as an over-the-counter medication only for veterinary use.
Another local parent, Precious Mudenge, said parents were not given the opportunity to make informed decisions about the health of their children as they were left without adequate information to do this.
She added that while it may be public health policy to administer drugs to children en-masse from time to time, this time there was need for communication from the ministry since most parents are not even aware that bilharzia and intestinal worms are a public health threat.
Her views were echoed by another parent, Gerald Sikhosana, who said that parents were left dependent on rumour and speculation spreading through social media rather than getting facts from the ministry.
Some parents have expressed concern over the fact that the drugs were donated, wondering whether developed countries might be using Zimbabwe as a dumping ground for expired medicines.
Disease Control director Dr. Portia Manangazira said although the drugs were a donation, they are of good quality, saying they were reviewed by the Medical Council of Zimbabwe to ensure their acceptability.
According to the Ministry of Health and Child Care, a nationwide prevalence survey conducted in 2010 indicated that bilharzia and intestinal worms were widespread, affecting 57 of the country’s 63 districts.
The Permanent Secretary of Health, Dr. Gerald Gwinji, said his ministry will extend the programme by two days to enable more children to access treatment which is being administered by health professionals at schools and other health centers.
Gwinji said infants and school children are given medication in the form of one tablet taken orally once to help fight the bacteria that causes the two diseases.
According to the website of the U.S Mayo Clinic, a respected international health care provider, the drug is not licensed for use by humans in the UK, but may be prescribed. The same is true in the US. As an over-the-counter medicine, available for general use, the UK and U.S only allow the drug for use on animals.
The Ministry of Health and Child Care last week embarked on a mass drug administration exercise, hoping to distribute about 11.5 million donated praziquantel tablets to treat 4.3 million children for bilharzia and intestinal worms. The ministry hoped to be able to reach all children aged 1 to 15 in all of Zimbabwe’s 63 districts.
But some parents declined to let their children take the drugs, saying they needed more information about the drug and any possible side effects. They charge that they had received no information about the treatment program, learning about it only when ministry officials began showing up at area schools.
One Bulawayo parent, Emily Ndlovu, says she did not allow her son to have the drug administered to him at his school. She said she was not comfortable with her son taking praziquantel, mistakenly believing that the drug is banned in the UK. While the drug is not banned, it is true that it is available in the UK and the US only when prescribed by a doctor. It is available as an over-the-counter medication only for veterinary use.
Another local parent, Precious Mudenge, said parents were not given the opportunity to make informed decisions about the health of their children as they were left without adequate information to do this.
She added that while it may be public health policy to administer drugs to children en-masse from time to time, this time there was need for communication from the ministry since most parents are not even aware that bilharzia and intestinal worms are a public health threat.
Her views were echoed by another parent, Gerald Sikhosana, who said that parents were left dependent on rumour and speculation spreading through social media rather than getting facts from the ministry.
Some parents have expressed concern over the fact that the drugs were donated, wondering whether developed countries might be using Zimbabwe as a dumping ground for expired medicines.
Disease Control director Dr. Portia Manangazira said although the drugs were a donation, they are of good quality, saying they were reviewed by the Medical Council of Zimbabwe to ensure their acceptability.
According to the Ministry of Health and Child Care, a nationwide prevalence survey conducted in 2010 indicated that bilharzia and intestinal worms were widespread, affecting 57 of the country’s 63 districts.
The Permanent Secretary of Health, Dr. Gerald Gwinji, said his ministry will extend the programme by two days to enable more children to access treatment which is being administered by health professionals at schools and other health centers.
Gwinji said infants and school children are given medication in the form of one tablet taken orally once to help fight the bacteria that causes the two diseases.
According to the website of the U.S Mayo Clinic, a respected international health care provider, the drug is not licensed for use by humans in the UK, but may be prescribed. The same is true in the US. As an over-the-counter medicine, available for general use, the UK and U.S only allow the drug for use on animals.